
SL Paper 3
This question is about radioactive decay.
The half-life of Au-189 is 8.84 minutes. A freshly prepared sample of the isotope has an activity of 124Bq.
A nucleus of a radioactive isotope of gold (Au-189) emits a neutrino in the decay to a nucleus of an isotope of platinum (Pt).
In the nuclear reaction equation below, state the name of the particle X and identify the nucleon number \(A\) and proton number \(Z\) of the nucleus of the isotope of platinum.
\[_{\;79}^{189}Au \to _Z^APt + X + v\]
X:
A:
Z:
(i) Calculate the decay constant of Au-189.
(ii) Determine the activity of the sample after 12.0 min.
This question is about radioactive decay.
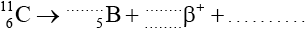
In a particular nuclear medical imaging technique, carbon-11 \((_{\;6}^{11}{\text{C}})\) is used. It is radioactive and decays through \({\beta ^ + }\) decay to boron (B).
The half-life of carbon-11 is 20.3 minutes.
Identify the numbers and the particle to complete the decay equation.
State the nature of the \({\beta ^ + }\) particle.
Outline a method for measuring the half-life of an isotope, such as the half-life of carbon-11.
State the law of radioactive decay.
Derive the relationship between the half-life \({T_{\frac{1}{2}}}\) and the decay constant \(\lambda \) , using the law of radioactive decay.
Calculate the number of nuclei of carbon-11 that will produce an activity of \(4.2 \times {10^{20}}{\text{ Bq}}\).
This question is about wave–particle duality.
A particle of mass \({\text{6.4}} \times {\text{1}}{{\text{0}}^{ - {\text{27}}}}{\text{ kg}}\) and charge \(3.2 \times {10^{ - 19}}{\text{ C}}\) is accelerated from rest through a potential difference of 25 kV.
Describe what is meant by the de Broglie hypothesis.
(i) Calculate the kinetic energy of the particle.
(ii) Determine the de Broglie wavelength of the particle.
This question is about nuclear physics and radioactive decay.
Define decay constant.
A sample of 1.6 mol of the radioactive nuclide radon-210 \(\left( {{}_{86}^{210}{\rm{Rn}}} \right)\) decays into polonium-206 \(\left( {{}_{84}^{206}{\rm{Po}}} \right)\) with the production of one other particle.
\[{}_{86}^{210}{\rm{Rn}} \to {}_{84}^{206}{\rm{Po + X}}\]
(i) Identify particle X.
(ii) The radioactive decay constant of radon-210 is 8.0×10–5s–1. Determine the time required to produce 1.1 mol of polonium-206.
Particle X has an initial kinetic energy of 6.2MeV after the decay in (b). In a scattering experiment, particle X is aimed head-on at a stationary gold-197 \(\left( {{}_{76}^{197}{\rm{Au}}} \right)\) nucleus.
Determine the distance of closest approach of particle X to the Au nucleus.
This question is about radioactive decay.
A nucleus of magnesium-23 decays forming a nucleus of sodium-23 with the emission of an electron neutrino and a β+ particle.
Outline why the existence of neutrinos was hypothesized to account for the energy spectrum of beta decay.
The decay constant for magnesium-23 is 0.061 s−1. Calculate the time taken for the number of magnesium-23 nuclei to fall to 12.5% of its initial value.
This question is about the wave nature of matter.
In 1927 Davisson and Germer tested the de Broglie hypothesis. They directed a beam of electrons onto a nickel crystal as shown in the diagram. The experiment was carried out in a vacuum.
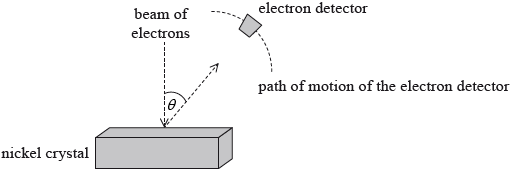
Describe wave-particle duality in relation to the de Broglie hypothesis.
The electrons were accelerated through a potential difference of 54 V. Show that the associated de Broglie wavelength for the electrons is about \(2 \times {10^{ - 10}}{\text{ m}}\).
The electron detector recorded a large number of electrons at a particular scattering angle \(\theta \). Explain why a maximum in the number of scattered electrons is observed at a particular angle.
This question is about the photoelectric effect.
In a photoelectric experiment, light of wavelength 450 nm is incident on a sodium surface. The work function for sodium is 2.4 eV.
(i) Calculate, in eV, the maximum kinetic energy of the emitted electrons.
(ii) The number of electrons leaving the sodium surface per second is 2 \( \times \) 1015. Calculate the current leaving the sodium surface.
The wavelength of the light incident on the sodium surface is decreased without changing its intensity. Explain why the number of electrons emitted from the sodium will decrease.
This question is about quantum physics.
Describe the de Broglie hypothesis.
An electron is accelerated from rest through a potential difference of 5.0 kV.
(i) Calculate the momentum of the electron after acceleration.
(ii) Calculate the wavelength of the electron.
(iii) Determine the energy of a photon that has the same wavelength as the electron in (b)(ii).
The momentum of the electron is known precisely. Deduce that all the information on its position is lost.
With reference to Schrödinger’s model, state the meaning of the amplitude of the wavefunction for the electron.
This question is about the photoelectric effect.
When light is incident on a clean metal surface, electrons can be emitted through the photoelectric effect.
Outline how the Einstein model is used to explain the photoelectric effect.
State why, although the incident light is monochromatic, the energies of the emitted electrons vary.
Explain why no electrons are emitted if the frequency of the incident light is less than a certain value, no matter how intense the light.
For monochromatic light of wavelength 620 nm a stopping potential of 1.75 V is required. Determine the minimum energy required to emit an electron from the metal surface.
This question is about energy level transitions.
Some of the electron energy levels for a hydrogen atom are shown.
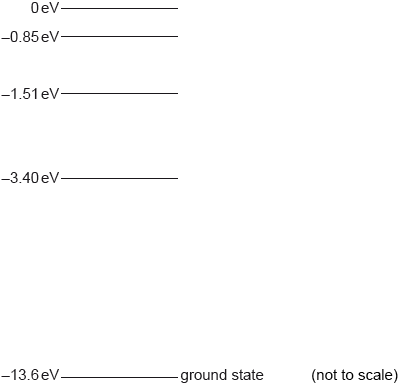
A hydrogen atom is excited to the \( - 1.51{\text{ eV}}\) level.
Monochromatic radiation is incident on gaseous hydrogen. All the hydrogen atoms are in the ground state. Describe what could happen to the radiation and to the hydrogen atoms if the incident photon energy is equal to
On the diagram, label using arrows all the possible transitions that might occur as the hydrogen atom returns to the ground state.
State the energy in eV of the maximum wavelength photon emitted as the hydrogen atom returns to the ground state.
10.2 eV.
9.0 eV.
This question is about radioactive decay.
Define the decay constant of a radioactive isotope.
Show that the decay constant \(\lambda \) is related to the half-life \({T_{\frac{1}{2}}}\) by the expression
\[\lambda {T_{\frac{1}{2}}} = \ln 2.\]
Strontium-90 is a radioactive isotope with a half-life of 28 years. Calculate the time taken for 65% of the strontium-90 nuclei in a sample of the isotope to decay.
This question is about wave–particle duality.
In the photoelectric effect, electrons are not emitted from the surface of a metal if the frequency of the incident light is below a certain value called the threshold frequency.
Light of frequency \(1.0 \times {10^{15}}{\text{ Hz}}\) is incident on the surface of a metal. The work function of the metal is \(3.2 \times {10^{ - 19}}{\text{ J}}\).
(i) Explain, with reference to the Einstein model of the photoelectric effect, the existence of the threshold frequency.
(ii) State, with reference to your answer in (a)(i), the reason why the threshold frequency is different for different metals.
(i) Show that the maximum kinetic energy of the emitted electrons is \(3.4 \times {10^{ - 19}}{\text{ J}}\).
(ii) Determine the de Broglie wavelength of the electrons in (b)(i).
This question is about nuclear energy levels and nuclear decay.
The isotope bismuth-212 undergoes α-decay to an isotope of thallium. In this decay a gamma-ray photon is also produced. The isotope potassium-40 undergoes β+ decay to an isotope of argon.
Outline how the
(i) α particle spectrum and the gamma spectrum of the decay of bismuth-212 give evidence for the existence of discrete nuclear energy levels.
(ii) β+ spectrum of the decay of potassium-40 led to the existence of the neutrino being postulated.
The isotope potassium-40 occurs naturally in many rock formations. In a particular sample of rock it is found that, out of the total number of argon plus potassium-40 atoms, 23% are potassium-40 atoms.
Determine the age of the rock sample. The decay constant for potassium-40 is 5.3×10–10yr–1.
This question is about plutonium as a power source.
Plutonium (\({}_{94}^{238}{\rm{Pu}}\)) decays by alpha emission. The energy of the alpha particle emitted is 8.8×10–13J. The decay constant of plutonium-238 is 8.1×10–3yr–1.
Define decay constant.
Plutonium-238 is to be used as a power source in a space probe.
(i) Determine the initial activity of plutonium such that the power released by plutonium is 6.0 W.
(ii) The power source becomes useless when the power released decreases to 4.0 W. Determine the time, in years, for which the power source can be used in the space probe.
This question is about radioactive decay.
A nuclide of the isotope potassium-40 \(\left( {{}_{19}^{40}{\rm{K}}} \right)\) decays into a stable nuclide of the isotope
argon-40 \(\left( {{}_{18}^{40}{\rm{Ar}}} \right)\). Identify the particles X and Y in the nuclear equation below.
\[{}_{19}^{40}{\rm{K}} \to {}_{18}^{40}{\rm{Ar + X + Y}}\]
The half-life of potassium-40 is 1.3×109yr. In a particular rock sample it is found that 85 % of the original potassium-40 nuclei have decayed. Determine the age of the rock.
State the quantities that need to be measured in order to determine the half-life of a long-lived isotope such as potassium-40.
This question is about radioactive decay.
Sodium-22 undergoes β+ decay.
Identify the missing entries in the following nuclear reaction.
\[{}_{11}^{22}{\rm{Na}} \to {}_ \ldots ^{22}{\rm{Ne}} + {}_ \ldots ^0e + {}_0^0 \ldots \]
Define half-life.
Sodium-22 has a decay constant of 0.27 yr–1.
(i) Calculate, in years, the half-life of sodium-22.
(ii) A sample of sodium-22 has initially 5.0 × 1023 atoms. Calculate the number of sodium-22 atoms remaining in the sample after 5.0 years.
This question is about the photoelectric effect.
Describe the concept of a photon.
In the photoelectric effect there exists a threshold frequency below which no emission of photoelectrons takes place.
Outline how the
(i) wave theory of light is unable to account for this observation.
(ii) concepts of the photon and work function are able to account for this observation.
Light of wavelength 420 nm is incident on a clean metal surface. The work function of the metal is 2.0 eV.
Determine the
(i) threshold frequency for this metal.
(ii) maximum kinetic energy in eV of the emitted electrons.
This question is about the photoelectric effect and the de Broglie hypothesis.
When photons are incident on a lithium surface photoelectrons are emitted. The work function φ of lithium is 2.9 eV.
Define work function.
Determine the maximum wavelength of the photons that can cause photoemission.
Calculate the momentum of an electron that has the same de Broglie wavelength as the wavelength of the photons in (b).
This question is about radioactive decay.
Nitrogen-13 \(\left( {{}_7^{13}{\rm{N}}} \right)\) is an isotope that is used in medical diagnosis. The decay constant of nitrogen-13 is 1.2×10–3s–1.
(i) Define decay constant.
(ii) A sample of nitrogen-13 has an initial activity of 800 Bq. The sample cannot be used for diagnostic purposes if its activity becomes less than 150 Bq. Determine the time it takes for the activity of the sample to fall to 150 Bq.
(i) Calculate the half-life of nitrogen-13.
(ii) Outline how the half-life of a sample of nitrogen-13 can be measured in a laboratory.
Nitrogen-13 undergoes β+ decay. Outline the experimental evidence that suggests another particle, the neutrino, is also emitted in the decay.
This question is about radioactive decay.
Meteorites contain a small proportion of radioactive aluminium-26 \(\left( {_{{\text{13}}}^{{\text{26}}}{\text{Al}}} \right)\) in the rock.
The amount of \(_{{\text{13}}}^{{\text{26}}}{\text{Al}}\) is constant while the meteorite is in space due to bombardment with cosmic rays.
After reaching Earth, the number of radioactive decays per unit time in a meteorite sample begins to diminish with time. The half-life of aluminium-26 is \(7.2 \times {10^5}\) years.
Aluminium-26 decays into an isotope of magnesium (Mg) by \({\beta ^ + }\) decay.
\[_{{\text{13}}}^{{\text{26}}}{\text{Al}} \to _{\text{Y}}^{\text{X}}{\text{Mg}} + {\beta ^ + } + {\text{Z}}\]
Identify X, Y and Z in this nuclear decay process.
X:
Y:
Z:
Explain why the beta particles emitted from the aluminium-26 have a continuous range of energies.
State what is meant by half-life.
A meteorite which has just fallen to Earth has an activity of 36.8 Bq. A second meteorite of the same mass, which arrived some time ago, has an activity of 11.2 Bq. Determine, in years, the time since the second meteorite arrived on Earth.
This question is about the de Broglie hypothesis.
State the de Broglie hypothesis.
Determine the de Broglie wavelength of a proton that has been accelerated from rest through a potential difference of 1.2 kV.
Explain why a precise knowledge of the de Broglie wavelength of the proton implies that its position cannot be observed.
This question is about the photoelectric effect.
State what is meant by the photoelectric effect.
Light of frequency 8.7×1014Hz is incident on the surface of a metal in a photocell. The surface area of the metal is 9.0×10–6m2 and the intensity of the light is 1.1×10–3Wm–2.
(i) Deduce that the maximum possible photoelectric current in the photocell is 2.7 nA.
(ii) The maximum kinetic energy of photoelectrons released from the metal surface is 1.2 eV. Calculate the value of the work function of the metal.
This question is about neutrinos.
The spectrum of electron energies emitted in a typical β-decay is continuous. Describe how this observation led physicists to propose the existence of the particles now called neutrinos.
This question is about radioactive decay.
Nuclide X has a half-life that is estimated to be in the thousands of years.
Outline how the half-life of X can be determined experimentally.
A pure sample of X has a mass of 1.8 kg. The half-life of X is 9000 years. Determine the mass of X remaining after 25000 years.
This question is about radioactive decay.
Potassium-40 (K-40) is a radioactive isotope that occurs naturally in many different types of rock. A very small percentage of the isotope undergoes β+ decay to form an isotope of argon (Ar). Construct and complete the nuclear reaction equation for this decay.
Overall about 10% of a sample of K-40 will decay to argon. In a particular rock sample it is found that there are 1.6×1022 atoms of K-40 and 8.4×1021 atoms of argon. The half-life of K-40 is 1.2×109 yr. Estimate the time elapsed since the rock sample was formed.
This question is about the photoelectric effect.
In an experiment to investigate the photoelectric effect, light of frequency ƒ is incident on the metal surface A, shown in the diagram below. A potential difference is applied between A and B. The photoelectric current is measured by a sensitive ammeter. (Note: the complete electrical circuit is not shown.)
When the frequency of the light is reduced to a certain value, the current measured by the ammeter becomes zero. Explain how Einstein’s photoelectric theory accounts for this observation.
This question is about the photoelectric effect.
Monochromatic light of different frequencies is incident on a metal surface placed in a vacuum. As the frequency is increased a value is reached at which electrons are emitted from the surface. Below this frequency, no matter how intense the light, no electrons are emitted. Outline how the
(i) wave theory of light is unable to account for these observations.
(ii) Einstein model of the photoelectric effect is able to account for these observations.
The graph shows how the maximum kinetic energy EK of the ejected electrons in (a) varies with the frequency f of the incident light.
Use the graph to determine the
(i) Planck constant.
(ii) work function of the metal.
Show that electrons of energy 0.50 eV have a de Broglie wavelength of about 1.7×10–9m.
This question is about quarks and interactions.
Outline how interactions in particle physics are understood in terms of exchange particles.
Determine whether or not strangeness is conserved in this decay.
The total energy of the particle represented by the dotted line is 1.2 GeV more than what is allowed by energy conservation. Determine the time interval from the emission of the particle from the s quark to its conversion into the d \({\rm{\bar d}}\) pair.
The pion is unstable and decays through the weak interaction into a neutrino and an anti-muon.
Draw a Feynman diagram for the decay of the pion, labelling all particles in the diagram.
This question is about the photoelectric effect.
The diagram shows apparatus used to investigate the photoelectric effect.
When red light is incident on the metallic surface M the microammeter registers a current. Explain how a current is established in this circuit even though nothing joins M to C inside the tube.
The graph shows the variation with voltage V of the current I in the circuit.
The work function of the metallic surface M is 0.48 eV.
(i) Define work function.
(ii) State the maximum kinetic energy of an electron immediately after it has been emitted from M.
(iii) Calculate the energy of a photon incident on M.
(iv) The red light incident on M is now replaced by blue light. The number of photons incident on M per second is the same as in (b).
On the axes opposite, sketch a graph to show the variation with V of the current I.
This question is about the photoelectric effect.
The diagram shows the set up of an experiment designed to verify the Einstein model of the photoelectric effect.
The tungsten electrode is positive.
Explain how the maximum kinetic energy of electrons ejected from the positive electrode is determined.
Light of frequency f is shone onto the tungsten electrode in (a). The potential Vs for which the photoelectric current is zero is recorded for different values of f.
(i) Using the axes below, sketch a graph of how you might expect Vs to vary with f.
(ii) State the Einstein photoelectric equation in a form that relates Vs and f. Define, other than the electron charge, any other symbols that you might use.
(iii) Outline how a graph of Vs against f can be used to find the Planck constant and work function of tungsten.
The work function of tungsten is 4.5eV. Show that the de Broglie wavelength of an electron that has this energy is about 0.6nm.
This question is about radioactive decay.
Iodine-124 (I-124) is an unstable radioisotope with proton number 53. It undergoes beta plus decay to form an isotope of tellurium (Te).
State the reaction for the decay of the I-124 nuclide.
The graph below shows how the activity of a sample of iodine-124 changes with time.
(i) State the half-life of iodine-124.
(ii) Calculate the activity of the sample at 21 days.
(iii) A sample of an unknown radioisotope has a half-life twice that of iodine-124 and the same initial activity as the sample of iodine-124. On the axes opposite, draw a graph to show how the activity of the sample would change with time. Label this graph X.
(iv) A second sample of iodine-124 has half the initial activity as the original sample of iodine-124. On the axes opposite, draw a graph to show how the activity of this sample would change with time. Label this graph Y.
This question is about atomic energy levels.
Explain how atomic spectra provide evidence for the quantization of energy in atoms.
Outline how the de Broglie hypothesis explains the existence of a discrete set of wavefunctions for electrons confined in a box of length L.
The diagram below shows the shape of two allowed wavefunctions ѱA and ѱB for an electron confined in a one-dimensional box of length L.
(i) With reference to the de Broglie hypothesis, suggest which wavefunction corresponds to the larger electron energy.
(ii) Predict and explain which wavefunction indicates a larger probability of finding the electron near the position \(\frac{L}{2}\) in the box.
(iii) On the graph in (c) on page 7, sketch a possible wavefunction for the lowest energy state of the electron.